Biosimilar Interchangeability Checker
Results for
Important: The Purple Book shows FDA approval status, but state substitution rules vary. Always check your state's pharmacy laws.
The Purple Book isn’t a book you buy at a bookstore. It’s a live, searchable database run by the U.S. Food and Drug Administration (FDA) that tells you exactly which biological drugs are approved, which ones are biosimilars, and which ones can be swapped out like generics. If you’re a pharmacist, a prescriber, or even a patient trying to understand why your insulin or arthritis drug changed without a new prescription, the Purple Book is the official source you need to trust.
What Exactly Is the Purple Book?
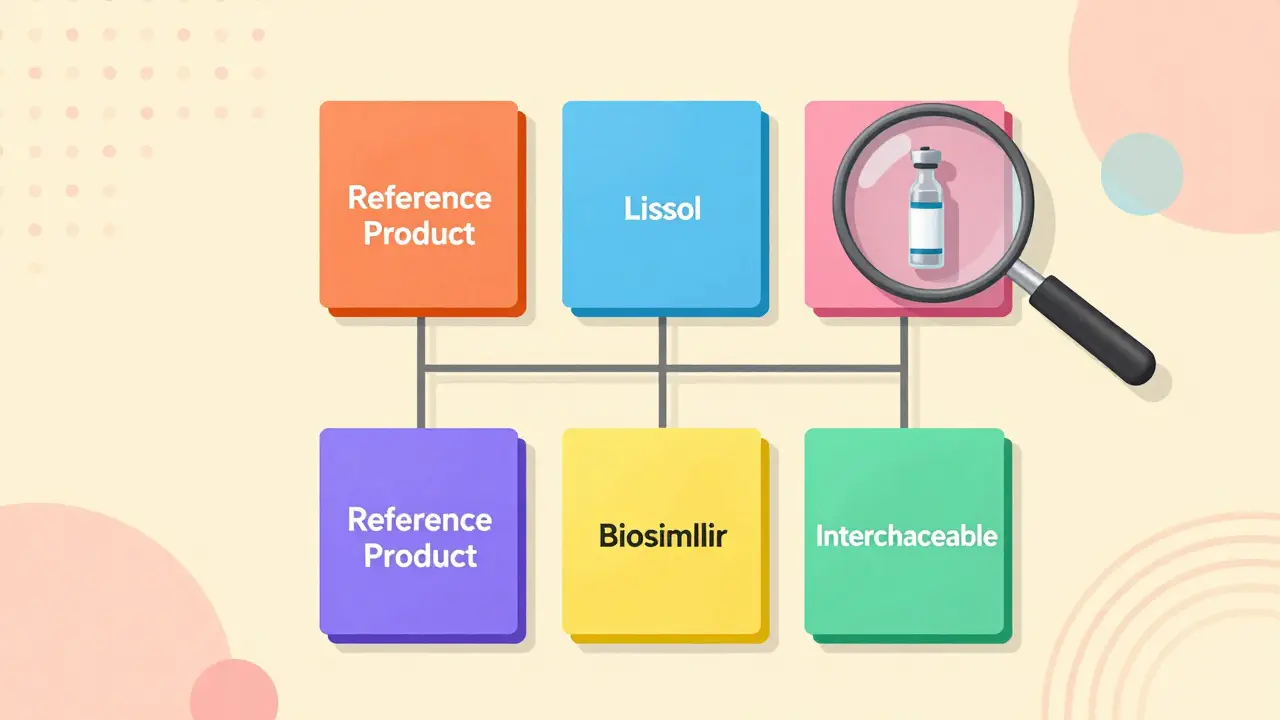
The Purple Book is the FDA’s official list of all licensed biological products in the U.S. That includes the original brand-name biologics, the biosimilars that copy them, and the rare few that are approved as interchangeable. Before 2020, this information was split into two separate lists-one for drugs managed by the Center for Drug Evaluation and Research (CDER), and another for biologics like vaccines and cell therapies handled by the Center for Biologics Evaluation and Research (CBER). Now, it’s one clean, searchable database that updates in real time.Think of it like a digital index card system for complex medicines. Each product card shows the brand name, the generic name, the date it was approved, and crucially, whether it’s a reference product (the original), a biosimilar, or an interchangeable biosimilar. The cards are color-coded: matching colors mean products are linked by biosimilarity or interchangeability. If you see two cards with the same color, one is likely the original and the other is its copy.
Biosimilars vs. Interchangeable Biosimilars: The Key Difference
Not all biosimilars are created equal. All interchangeable biosimilars are biosimilars-but only a small number ever reach the interchangeable status. Here’s why that matters.A biosimilar is a biological product that’s highly similar to an FDA-approved reference product. There might be tiny differences in inactive ingredients, but no clinically meaningful differences in safety, purity, or how well it works. That’s the standard for approval. The FDA requires extensive lab tests, animal studies, and human clinical trials to prove this.
An interchangeable biosimilar goes further. To earn that label, the manufacturer must prove that switching back and forth between the biosimilar and the original drug doesn’t increase risk or reduce effectiveness. That means if a patient takes the reference product for six months, then switches to the biosimilar for three months, then switches back-there should be no drop in results or spike in side effects. This is called a “switching study,” and it’s not required for regular biosimilars.
As of November 2023, only seven biosimilars in the U.S. had received this interchangeability designation. They include two insulin products, three for inflammatory diseases like rheumatoid arthritis, and two for eye conditions. That’s out of dozens of approved biosimilars. The FDA makes it clear: interchangeability doesn’t mean the drug is better. It just means it can be substituted without a doctor’s new order.
Why Does Interchangeability Matter to Pharmacists?
This is where things get real for people behind the pharmacy counter. In most states, pharmacists can substitute a generic drug for a brand-name pill without asking the doctor. But for biologics, the rules are different.Even if the FDA says a biosimilar is interchangeable, the pharmacist still has to follow state law. As of 2023, 47 states and Puerto Rico allow pharmacists to swap an interchangeable biosimilar for the original without contacting the prescriber. But in those states, there are often extra steps: they must notify the doctor, document the switch, and sometimes inform the patient.
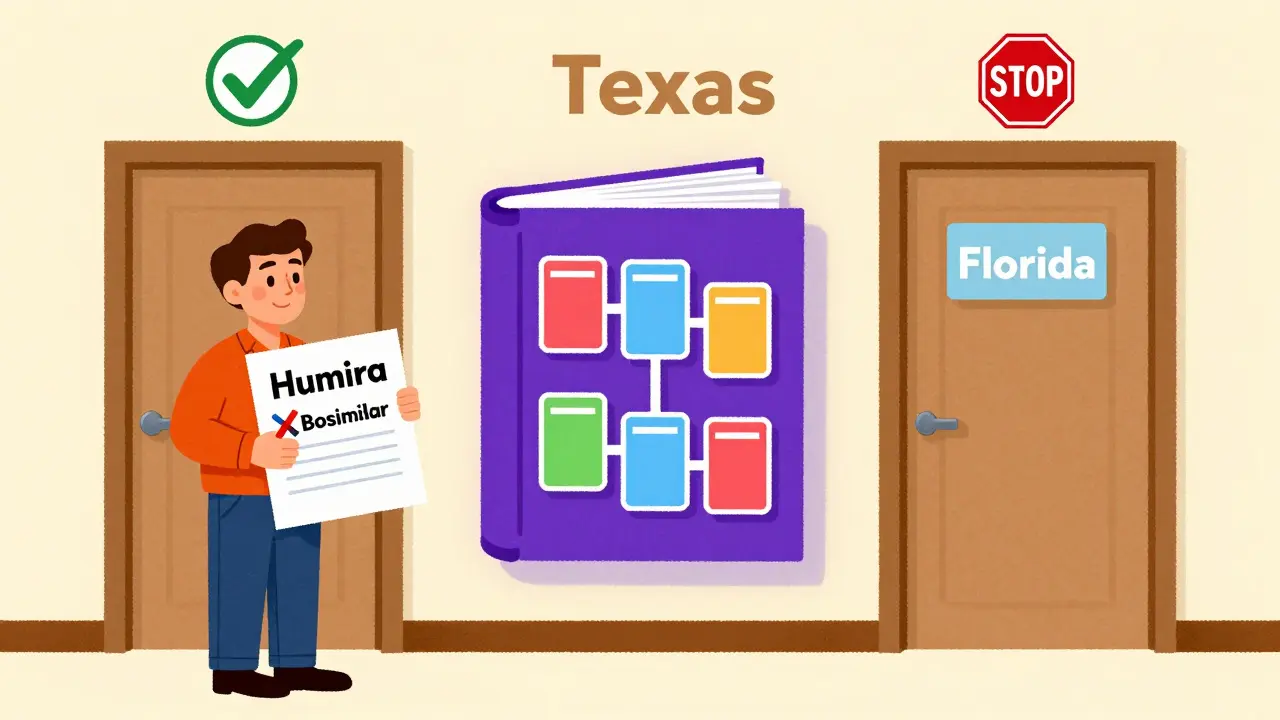
In the other three states, substitution is either banned or requires the prescriber to specifically allow it on the prescription. That means a patient in New York might get a biosimilar automatically, while a patient in California might need a new script-even if both are using the exact same FDA-approved product.
This patchwork system creates confusion. A pharmacist in Texas might stock three interchangeable insulin biosimilars, but if the patient moves to Florida, the rules change. The Purple Book doesn’t tell you what your state allows-it only tells you what the FDA approved. You still need to check your state pharmacy board’s rules.

How to Use the Purple Book Like a Pro
The Purple Book isn’t built for casual browsing. It’s a tool for professionals. Here’s how to use it effectively:- Go to the FDA’s Purple Book page on fda.gov.
- Use the search bar to look up a brand name, like "Humira" or "Enbrel."
- Click on the reference product card. You’ll see all the biosimilars and interchangeable products linked to it under the same color group.
- Look for the "351(k) Interchangeable" tag next to any product. That’s your green light for substitution-at least at the federal level.
- Check the "Date Licensed" to see how long the product has been on the market. Older products have more real-world data.
Pro tip: Don’t rely on the product name alone. Two biosimilars might have similar names but be linked to different reference products. Always match the product card color and the FDA designation.
What the Purple Book Doesn’t Tell You
The Purple Book is powerful, but it’s not a complete guide. It won’t tell you:- What your insurance will cover
- Which biosimilar your pharmacy stocks
- How much a patient will pay out of pocket
- Whether a prescriber is willing to switch a patient
It also doesn’t label "unbranded biologics" as interchangeable-even though the FDA considers them equivalent. These are products without a brand name, often made by the same company as the original, and they’re not always listed the same way.
And while the FDA updates the Purple Book regularly, there’s always a lag. A product might be approved last week but won’t show up until the next database refresh. Always check the "Last Updated" date at the top of the page.
Why This Matters for Patients
Biologics are expensive. Humira, for example, costs over $7,000 a month without insurance. Biosimilars can cut that cost by 30% to 50%. Interchangeable biosimilars can drop it even further because pharmacies can swap them without extra paperwork.But patients don’t always know they’re getting a copy. If your doctor prescribes "adalimumab," you might get a biosimilar without being told. That’s legal and safe-but it’s still a big change. The Purple Book helps you verify that the drug you’re getting is approved and backed by science.
Patients with chronic conditions-like Crohn’s disease, psoriasis, or diabetes-often stay on biologics for years. Knowing whether their medication is interchangeable gives them confidence that switching won’t break their treatment. The FDA’s data shows no drop in effectiveness after multiple switches. That’s not just theory. It’s proven in real patients.
The Future of Biosimilars in the U.S.
The number of biosimilars approved each year is rising. In 2024, more companies are applying for interchangeability status than ever before. Insulin, monoclonal antibodies, and even some cancer drugs are in the pipeline.But progress is slow. The U.S. lags behind Europe and Canada in biosimilar adoption. Why? High legal costs, patent battles, and payer restrictions all play a role. The Purple Book is meant to cut through that noise. It’s a transparent tool that puts the facts in one place.
As more biosimilars hit the market, the Purple Book will become even more essential. It’s not just a reference-it’s the foundation for safer, cheaper, and more predictable care. For pharmacists, it’s the rulebook. For doctors, it’s the evidence base. For patients, it’s the reassurance they’re getting a drug that works.
Is the Purple Book only for pharmacists?
No. While pharmacists use it daily to decide what they can substitute, doctors, nurses, insurance companies, and even patients can use it to verify whether a biosimilar is FDA-approved and whether it’s interchangeable. It’s the official source for anyone needing clarity on biological drugs.
Can I substitute a biosimilar without a doctor’s permission?
Only if the biosimilar has an FDA interchangeability designation AND your state allows substitution without prescriber approval. As of 2023, 47 states and Puerto Rico permit this. But you must check your state’s pharmacy laws-some require notification or documentation even if substitution is allowed.
Are interchangeable biosimilars safer than regular biosimilars?
No. The FDA is clear: interchangeability doesn’t mean the drug is safer or more effective. It only means that switching between the biosimilar and the original drug won’t cause harm or reduce results. Both types are equally safe and effective for initial use.
Why are so few biosimilars approved as interchangeable?
Because proving interchangeability requires extra clinical trials-specifically, studies showing that multiple switches between the biosimilar and the original drug don’t affect safety or effectiveness. These studies are expensive and time-consuming. Many manufacturers skip them because they can still sell their biosimilars as non-interchangeable, especially if the prescriber chooses them.
How often is the Purple Book updated?
The FDA updates the Purple Book regularly, usually within days of a new approval or designation. But there can be a delay of up to a week or two. Always check the "Last Updated" date on the site before relying on it for clinical decisions.
Does the Purple Book include biologics for veterinary use?
No. The Purple Book only includes human biological products approved by the FDA. Veterinary biologics are regulated by the USDA and are not listed here.
Can I use the Purple Book to find cheaper alternatives to my expensive biologic?
Yes. Search your current biologic by brand name, and you’ll see all approved biosimilars and interchangeable versions linked to it. You can then ask your doctor or pharmacist if one of those alternatives is right for you. Many are significantly cheaper and just as effective.



Windie Wilson
12 January 2026 - 01:51 AM
So the Purple Book is basically the FDA’s way of saying ‘here’s your biologic Tinder profile’ - swipe right if you’re interchangeable, left if you’re just a copycat. 🙃
Jose Mecanico
14 January 2026 - 01:08 AM
For those of us who actually work with these drugs daily, this is the only source we trust. The color-coding alone saves hours of cross-referencing old PDFs. Real game-changer.
Jessica Bnouzalim
15 January 2026 - 04:17 AM
OMG I JUST REALIZED I’VE BEEN GETTING A BIOSIMILAR FOR MY ANTI-TNF FOR 8 MONTHS AND NO ONE TOLD ME???!!! I’M FINE THOUGH??!! THE DRUG WORKS JUST AS WELL AND I SAVED $4K??!!
Sumit Sharma
16 January 2026 - 16:33 PM
Interchangeability requires demonstrating pharmacokinetic equivalence across multiple switch cycles, which demands Phase III clinical trials with non-inferiority endpoints. The regulatory burden is nontrivial, and many sponsors opt out due to cost-benefit analysis. This is not a failure of the system-it’s rational risk management.
Jay Powers
18 January 2026 - 03:57 AM
It's wild how much confusion there is around this stuff. I've seen patients panic thinking their meds changed when it's just a biosimilar. The Purple Book should be linked right on every prescription portal. Simple fix.
Katherine Carlock
18 January 2026 - 04:23 AM
My pharmacist switched me to a biosimilar last month without even asking. I didn’t care as long as it worked. Now I just ask if it’s interchangeable before they hand it over. Easy peasy.
Sona Chandra
18 January 2026 - 04:25 AM
Why are we even letting pharmacists swap biologics at all? This is not like aspirin. These are complex molecules. The FDA is rushing this to appease Big Pharma. Patients are guinea pigs.
Lauren Warner
19 January 2026 - 07:09 AM
Let’s not romanticize the Purple Book. It doesn’t reflect real-world formulary restrictions, prior authorizations, or payer-driven substitution policies. It’s a technical document with zero influence on actual access. You’re not empowered-you’re just informed.
Craig Wright
21 January 2026 - 03:09 AM
In the UK, we have the Green Book. It’s far more comprehensive, includes pricing, and mandates pharmacist notification. The U.S. system feels like a beta version. Why are we lagging behind Europe in transparency and regulation?
Lelia Battle
22 January 2026 - 00:00 AM
It’s fascinating how we treat biological drugs as if they’re interchangeable parts. But they’re not. They’re living molecules shaped by complex processes. The idea that we can swap them like Lego blocks speaks more to our desire for simplicity than to biological reality.
Rinky Tandon
23 January 2026 - 17:39 PM
Only 7 interchangeable biosimilars? Pathetic. The FDA is being manipulated by patent trolls and pharma lobbyists. The science is there. The cost savings are undeniable. This is corporate sabotage disguised as regulatory caution.
Ben Kono
23 January 2026 - 19:12 PM
Wait so if I’m on Humira and my doc prescribes adalimumab and I get a biosimilar… that’s fine right? I mean the Purple Book says it’s linked so it’s good right? No need to overthink it