When a medicine batch is released, someone has to say yes-or no. That decision shouldn’t come from the person trying to meet production targets. It should come from someone whose only job is to protect the patient. That’s the role of a quality assurance unit (QU), and its independence isn’t a best practice-it’s a legal requirement in regulated industries like pharmaceuticals and nuclear energy.
What Exactly Is a Quality Assurance Unit?
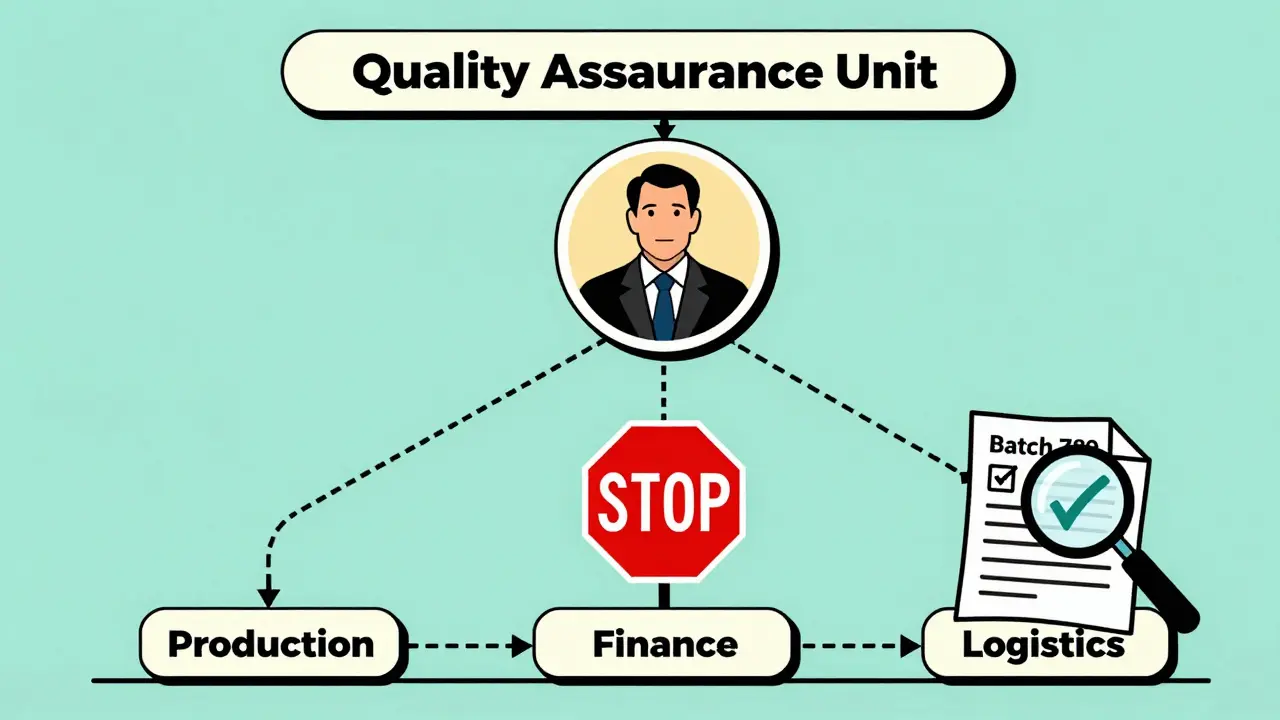
A quality assurance unit is a formally recognized team within a manufacturing facility that has the authority to approve or reject every step of production. This includes raw materials, packaging, in-process checks, and final product release. Unlike a quality control lab that tests samples, the QU oversees the entire system: procedures, records, audits, and trends. Their job isn’t to fix problems after they happen-it’s to stop them before they reach the patient. The FDA made this crystal clear in its 2006 guidance: the QU must be independent from production. That means no shared managers, no shared budgets, and no reporting up through the plant supervisor. The person who signs off on a batch shouldn’t be the same person who’s being pressured to hit daily output goals. The stakes are too high. One contaminated batch can kill. One falsified record can trigger a recall that costs millions and destroys trust.Why Independence Isn’t Optional
Think of it like a fire alarm. You don’t let the person who started the fire also decide when to sound the alarm. Yet, in many companies, that’s exactly what happens. When quality reports to production, decisions get blurred. A manager might delay a quality hold because “we’re behind schedule.” Or they might skip an investigation because “the batch looked fine.” FDA warning letters from 2023 to 2025 show a clear pattern: 68% of them cite failures in QU independence. In one case, a warehouse manager contacted the quality unit directly to get a batch released-bypassing proper channels. In another, the same person signed off as both production lead and quality lead. That’s not efficiency. That’s a violation. The data doesn’t lie. Facilities with truly independent QUs have 37% fewer critical failures during inspections, according to the IAEA. They resolve deviations 28% faster. And they’re far less likely to get flagged for data integrity issues-something that’s behind 63% of FDA warning letters in pharma.How Independence Works in Practice
In a compliant facility, the quality assurance unit doesn’t just sit in an office. They’re embedded in the system-but not part of the machine. Here’s how it looks on paper:- The QU reports directly to the CEO or the Board of Directors-not to the plant manager.
- They have their own budget, separate from production.
- They can halt production at any time without approval from anyone in manufacturing.
- They review and approve every procedure, change, and batch record before it’s executed.
- They conduct internal audits and trend analyses, not just batch-by-batch checks.
What Happens When Independence Fails
It doesn’t take long for things to go wrong. In 2024, a small pharma company with fewer than 50 employees tried to combine production and quality roles during a restructuring. Within three months, two critical deviations went uninvestigated. One batch was released with incorrect labeling. Patients got the wrong dosage. The FDA issued a warning letter. The company lost its license for six months. This isn’t rare. FDA data shows 42% of warning letters to small facilities cite QU independence failures. In larger companies, it’s only 18%. Why? Because small teams can’t afford separate staff. They try to cut corners. But cutting corners on quality isn’t saving money-it’s gambling with lives. Even in bigger companies, pressure builds. A 2025 survey of 312 quality professionals found that 57% reported being asked to “expedite batch reviews” during peak production. Thirty-three percent said their QU didn’t have real authority to stop production-even though regulations say they must.How to Build a Real Quality Assurance Unit
You can’t just hire someone and call them “Head of Quality.” You have to build a system. Start with structure:- Define the QU’s reporting line. It must go straight to the top-CEO, COO, or Board. No middle managers.
- Give them budget control. They need money for audits, training, and tools-without asking production for approval.
- Document their authority. Write it down. Publish it. Train everyone on it.
- Set a minimum staffing ratio. ISPE recommends 8-12% of total manufacturing staff. If you have 100 production workers, you need at least 8-12 people in quality.
- Train them in GMP, statistical process control, and conflict resolution. The average QU staff member has over 8 years of experience.

The Digital Challenge: AI and Real-Time Decisions
New technology is changing the game. AI systems now make real-time decisions on production lines-adjusting temperature, flow rates, even rejecting batches automatically. So where does the QU fit in? The FDA’s 2025 draft guidance on digital manufacturing says independence must still be maintained. But now, it’s not just about who reports to whom. It’s about who controls the algorithm. If the AI is trained by the production team, it might learn to prioritize speed over safety. The QU needs to audit the model. They need to validate the data. They need to have the final say on whether the AI’s decisions are acceptable. This isn’t science fiction. It’s happening now. And companies that treat AI as a black box are setting themselves up for failure.Global Standards: US vs. Europe vs. the Rest
The U.S. is strict. The FDA says: no shared reporting lines. No exceptions. Europe’s EMA is a little more flexible. They allow integrated structures-but only if there are “effective mechanisms” to guarantee independence. That sounds good, but in practice, it’s harder to prove. Many companies use it as a loophole. Globally, compliance varies. In the U.S., 82% of facilities meet QU independence standards. In emerging markets, it’s only 67%. The gap isn’t about knowledge-it’s about enforcement. That’s why third-party oversight services are growing fast. Small companies that can’t afford a full QU are hiring external auditors to act as their independent oversight body. The market for these services is growing at 14.2% a year.Final Thought: It’s Not About Cost. It’s About Trust.
Some managers argue that separating quality from production slows things down. They point to cases where integrated teams resolved issues 22% faster. But here’s the catch: those same teams had 17% more borderline compliance decisions. That means more batches that barely passed. More risk. More chance of a hidden defect slipping through. In the end, patients don’t care how fast your line runs. They care if the medicine works. And regulators don’t care how efficient you are. They care if you followed the rules. Independent quality oversight isn’t a cost center. It’s your insurance policy. It’s your reputation. It’s the reason people still trust your brand when something goes wrong. The data is clear. The regulations are clear. The moral obligation is clear. If you’re in manufacturing-especially in pharma or any high-risk industry-your quality unit must be independent. Not because it’s trendy. Not because it’s nice to have. Because if it’s not, someone will get hurt.Can a production manager also serve as the quality assurance lead?
No. Regulatory agencies like the FDA and EMA explicitly prohibit this. When one person controls both production targets and quality decisions, conflicts of interest are inevitable. FDA warning letters from 2023-2025 show that 68% of independence violations involve this exact scenario. Even if the person is well-intentioned, the system is designed to fail under pressure.
How small can a quality assurance unit be?
There’s no legal minimum, but ISPE recommends 8-12% of total manufacturing staff. A facility with 50 workers should have at least 4-6 dedicated quality staff. Smaller teams often lead to "rubber stamping"-where batch records are approved without proper review. FDA data shows facilities with a QU-to-production ratio below 1:15 have 3.2 times more repeat deviations.
What happens if the quality unit doesn’t have authority to reject a batch?
The facility is not compliant. Under 21 CFR 211.22, the quality unit must have the legal authority to reject components, containers, labeling, and finished products. If they can’t do that, they’re not a true quality assurance unit-they’re an advisory group. Regulatory agencies will issue warning letters, and in severe cases, shut down operations until the structure is fixed.
Do small manufacturers have to hire a full-time quality unit?
Not necessarily. Many small manufacturers use third-party quality oversight services. These external firms act as the independent quality unit, conducting audits, reviewing records, and approving batches on behalf of the company. This is a growing market-up 14.2% annually-and it’s a legitimate way to meet regulatory requirements without hiring full-time staff.
Is QU independence required outside of pharmaceuticals?
Yes. While most strict rules apply to pharma and medical devices, nuclear energy, aerospace, and food manufacturing also require independent oversight. The IAEA mandates it for nuclear plants. The FAA requires it for aircraft parts. Even ISO 9001 recommends separation, though it doesn’t enforce it like the FDA does. If your product affects safety, independence matters.