DBS Eligibility Checker
This tool helps assess if you might qualify for Deep Brain Stimulation (DBS) based on criteria from the article. Important: This is not medical advice. Consult your neurologist for professional assessment.
Results will appear here...
When faced with the daily ups and downs of Parkinson’s disease, many patients wonder if there’s a solution beyond medication. Parkinson's disease is a progressive neurological disorder characterized by tremor, stiffness, slowness of movement, and a host of non‑motor symptoms such as sleep problems and mood changes. While drugs like levodopa can smooth the ride for a while, they often lose effectiveness over time and bring side effects. That’s where Deep Brain Stimulation (DBS) steps in as a surgical option that can restore function and improve quality of life.
What Is Deep Brain Stimulation?
Deep Brain Stimulation is a neurosurgical procedure that delivers controlled electrical pulses to specific brain regions involved in motor control. The goal is to modulate abnormal neural activity that causes the hallmark motor symptoms of Parkinson’s.
Typical DBS systems consist of three components:
- Implanted electrodes that sit in the target nucleus (most commonly the Subthalamic Nucleus or Globus Pallidus Interna).
- A pulse generator, often called a neurostimulator, implanted under the collarbone.
- Connecting leads that run beneath the skin to link the electrodes to the pulse generator.
Patients can adjust settings non‑invasively using a handheld programmer, allowing clinicians to fine‑tune therapy as symptoms evolve.
How DBS Works: The Science Behind the Stimulation
Parkinson’s disease stems from the loss of dopamine‑producing cells in the substantia nigra, which throws the basal ganglia’s circuitry out of balance. By delivering high‑frequency electrical impulses to the Subthalamic Nucleus (STN) or Globus Pallidus Interna (GPi), DBS essentially “re‑boots” the circuitry, reducing excessive firing patterns.
Research shows that STN‑DBS can lower the required dose of levodopa by up to 50%, cutting medication‑related dyskinesias. GPi‑DBS, on the other hand, tends to be a better fit for patients whose dominant problem is troublesome involuntary movements.
Who’s a Good Candidate?
Not everyone with Parkinson’s qualifies for DBS. Ideal candidates generally meet these criteria:
- Diagnosed with Parkinson’s disease for at least four years.
- Experiencing motor fluctuations or dyskinesias that are not well‑controlled by medication.
- Responds positively to levodopa (i.e., a clear improvement in motor scores when on medication).
- No severe cognitive decline, uncontrolled psychiatric illness, or contraindicating medical conditions.
Neurologists often perform a “levodopa challenge” test to confirm that the brain still reacts to dopamine, a key predictor of DBS success.
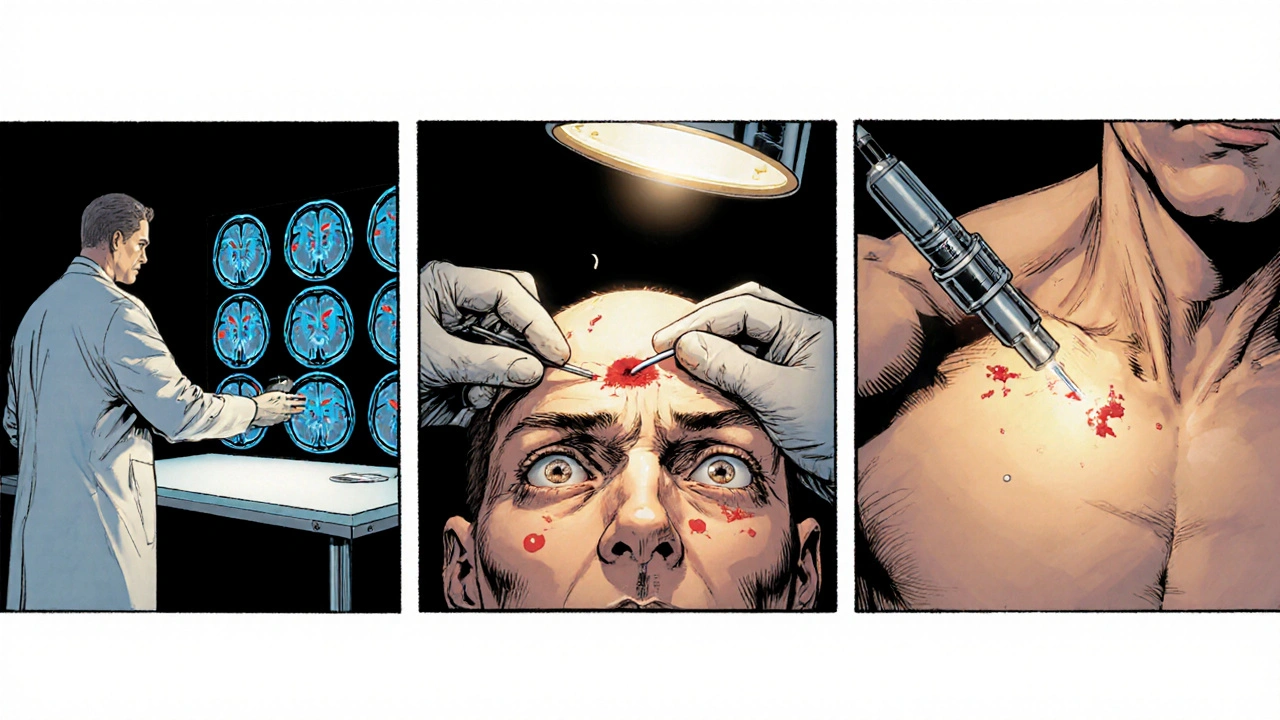
The Surgical Journey: Step‑by‑Step
DBS surgery can be broken into three main phases: planning, implantation, and programming.
- Pre‑operative imaging: High‑resolution MRI and CT scans map the target nucleus. Some centers use diffusion tensor imaging to visualize neural pathways.
- Electrode placement: Under local anesthesia (so the patient can stay awake and give feedback), a tiny burr hole is drilled. Surgeons then insert the electrode into the chosen nucleus. Intra‑operative micro‑electrode recordings confirm accurate positioning.
- Pulse generator implantation: Usually performed a few days later under general anesthesia, the neurostimulator (e.g., the Medtronic Activa system) is tucked under the skin near the collarbone and connected to the leads.
- Post‑operative programming: Weeks after surgery, clinicians adjust voltage, pulse width, and frequency to achieve optimal symptom control while minimizing side effects.
Because the patient is awake during electrode placement, any adverse sensations can be reported instantly, increasing safety.
Benefits You Can Expect
When DBS works as intended, patients often notice:
- Reduced tremor and rigidity.
- Fewer motor fluctuations (the “wear‑off” phenomenon).
- Lower medication doses, which means fewer nausea, dizziness, and dyskinesias.
- Improved gait and balance in many cases.
Importantly, these improvements are reversible-turn the device off, and symptoms revert to their pre‑DBS state, allowing clinicians to tweak settings without permanent brain alteration.
Risks and Common Concerns
No surgery is risk‑free. Potential complications include:
- Infection at the generator pocket (about 3‑5% of cases).
- Lead migration or breakage, which may require revision surgery.
- Bleeding or stroke, though this is rare (<1%).
- Side effects from stimulation such as speech difficulty, vision changes, or mood swings, usually manageable by adjusting parameters.
Long‑term device longevity typically ranges from 3 to 5 years, after which the battery can be replaced surgically.
DBS vs. Traditional Medical Therapy: A Quick Comparison
| Criterion | Levodopa Therapy | Deep Brain Stimulation |
|---|---|---|
| Invasiveness | Non‑invasive (oral medication) | Surgical (brain electrodes + implanted generator) |
| Motor symptom control | Effective early, less so over time | Consistently improves tremor, rigidity, and off‑time |
| Side‑effect profile | Medication‑induced dyskinesias, nausea, orthostatic hypotension | Device‑related infection, stimulation‑induced speech or mood changes |
| Adjustability | Dose changes only | Programmable parameters, non‑surgical adjustments |
| Long‑term sustainability | Declining efficacy after 5‑7 years | Benefits can last a decade or more with proper management |
This table highlights why many neurologists recommend DBS once medication starts to wear off or produce troublesome side effects.

Living with a DBS System: Daily Life and Follow‑Up
After the initial programming phase, patients typically see a neurologist every 3-6 months. Adjustments are common, especially as the disease evolves.
- Battery checks: Modern rechargeable systems can last 10+ years with regular charging.
- Activity considerations: Most patients can resume normal activities-including driving-once cleared by their physician.
- Travel tips: Carry a spare programmer and keep the device’s ID card handy for airport security.
Support groups and rehab programs (physical therapy, speech therapy) complement DBS by maximizing functional gains.
Future Directions: Advanced Targets and Closed‑Loop Systems
Researchers are exploring “closed‑loop” DBS, where sensors detect abnormal brain activity in real time and automatically adjust stimulation. Early trials targeting the pedunculopontine nucleus show promise for gait freezing, a symptom poorly addressed by conventional DBS.
Additionally, MRI‑compatible leads now allow doctors to image the brain with the device in place, improving long‑term management.
Quick Checklist Before Deciding on DBS
- Confirm positive response to levodopa.
- Assess cognitive and psychiatric health.
- Identify a multidisciplinary team: neurologist, neurosurgeon, neuropsychologist.
- Discuss device brand options (e.g., Medtronic Activa, Boston Scientific Vercise).
- Plan for post‑operative programming visits.
- Consider insurance coverage and out‑of‑pocket costs.
Frequently Asked Questions
How long does the DBS surgery take?
The electrode implantation usually lasts 2‑3 hours. Adding the pulse‑generator implantation adds another 1‑2 hours, so total operative time is about 4‑5 hours.
Is DBS reversible?
Yes. The device can be turned off at any time, and the hardware can be surgically removed if necessary. Symptoms typically return to their pre‑DBS level after removal.
What are the most common side effects?
Mild speech slurring, temporary tingling, or mood changes can occur but are usually corrected by adjusting stimulation settings. Infections occur in about 3‑5% of patients.
Can I still take levodopa after DBS?
Absolutely. DBS is an adjunct, not a replacement. Most patients continue a reduced dose of levodopa to manage residual symptoms.
How often does the battery need replacement?
Non‑rechargeable batteries last 3‑5 years. Rechargeable models can go 10 years or more with regular charging, and the generator can be swapped in a minor outpatient procedure.
Is DBS covered by insurance in Canada?
Provincial health plans, including Alberta Health, generally cover DBS when medically indicated, though prior authorization and specialist documentation are required.
Deep Brain Stimulation offers a powerful, adjustable tool for people living with Parkinson’s disease. By understanding the procedure, weighing benefits against risks, and partnering with an experienced care team, patients can make an informed choice that may transform daily life.







Richard O'Callaghan
17 October 2025 - 01:59 AM
I heard you had the DBS implant last month and it's pretty wild how fast you bounce back, it must feel like a whole new world but don't forget the meds still have a role, its not a magic fix and sometimes the battery runs out sooner than expected.
Just a heads up, the scar can get infected if you ignore the skin care routine.
Alexis Howard
28 October 2025 - 15:44 PM
Sure DBS sounds hightech but you could just up the levodopa dose and avoid surgery.
Steve Holmes
9 November 2025 - 06:29 AM
Man, the way DBS targets the subthalamic nucleus is fascinating, especially when you think about how those high‑frequency pulses basically re‑wire the basal ganglia, and the fact that patients can dial the settings from home is a game‑changer, plus the reduction in dyskinesia is often dramatic, right?
Tom Green
20 November 2025 - 21:14 PM
Absolutely, Steve! The programmable aspect really empowers patients-think of it as customizing a personal soundtrack for movement. While the tech is cutting‑edge, the real success hinges on multidisciplinary follow‑up, so keep those rehab sessions in the loop.
Kate Marr
2 December 2025 - 11:59 AM
Totally agree, Tom! 🇺🇸 Our surgeons are among the best in the world, and with DBS we’re showing that American innovation can outpace the rest. Keep the optimism high! 🚀
James Falcone
14 December 2025 - 02:44 AM
Can't argue with that-our hospitals have the top doctors and the best equipment, so choosing DBS here is a no‑brainer.
Frank Diaz
25 December 2025 - 17:29 PM
What truly matters is the underlying philosophy of meddling with brain circuitry; one must ask whether we are healing or merely masking the existential decay that Parkinson's reveals about the fragility of the self.
Valerie Vanderghote
6 January 2026 - 08:14 AM
I have to say, reading that article reminded me of the countless nights I stayed up waiting for my own father’s tremor to subside. The mechanics of deep brain stimulation are not just a medical marvel but a cultural signal that we are willing to rewrite our neural destiny. Every electrode placed in the subthalamic nucleus feels like a tiny rebellion against the inevitability of degeneration. Yet the procedure also forces us to confront the cold reality that a battery will eventually need replacement, adding another layer of dependency. The programming sessions, with their endless clicks and adjustments, become a ritual that blurs the line between therapy and obsession. In my experience, the most profound improvements often come after the patient learns to trust the device as an extension of their will. That trust is not given lightly; it is forged through sleepless weeks of trial and error, and through the whispered doubts that linger in the mind. Moreover, the postoperative care demands a network of specialists whose schedules rarely align, creating logistical nightmares that few anticipate. The financial burden, too, cannot be ignored, as insurance coverage varies wildly and out‑of‑pocket costs can be staggering. Some families view the expense as an investment in quality of life, while others see it as an unnecessary extravagance. The ethical debate surrounding invasive brain modulation continues to evolve, especially as technology pushes boundaries faster than regulations can keep up. Still, for many patients the reduction in medication side effects alone justifies the risk, offering a glimpse of a steadier, more manageable future. One should also remember that DBS does not cure Parkinson’s; it merely tempers the storm, leaving the underlying pathology untouched. This nuance is often lost in promotional brochures that promise a return to “normal” life. Personally, I find the balance between hope and realistic expectations to be the most delicate part of the journey. Ultimately, whether one chooses DBS or not, the decision must be rooted in an honest appraisal of both the benefits and the hidden costs.
Michael Dalrymple
17 January 2026 - 22:59 PM
Valerie, your thorough breakdown captures both the promise and the pitfalls of DBS beautifully; as a clinician I would add that a structured pre‑operative counseling session can mitigate many of the anxieties you mentioned, and ongoing multidisciplinary support often maximizes long‑term outcomes.
Kevin Adams
29 January 2026 - 13:44 PM
Behold! The stage of the brain lights up, an electrifying ballet of currents-do you feel the drama? The very soul of movement flickers, then steadies, as if destiny itself were being rewired...
Katie Henry
10 February 2026 - 04:29 AM
Esteemed community, I commend each individual who explores deep brain stimulation as a viable avenue; may your journey be marked by resilience, informed choices, and steadfast progress toward optimal health.