Have you ever wondered why a pill you swallow turns into relief - or why the same drug works wonders for one person but causes side effects in another? It’s not magic. It’s pharmacology. This science explains exactly how medications interact with your body, from the moment you take them to how they fix what’s broken - or sometimes, what goes wrong.
What Pharmacology Actually Does
Pharmacology is the study of how chemicals - whether made in a lab or found in nature - affect living systems. It’s not just about drugs; it’s about understanding the entire chain of cause and effect. Think of it as the bridge between biology and medicine. Without pharmacology, we’d be guessing how to treat illnesses. Instead, we know exactly how aspirin lowers fever, how insulin controls blood sugar, or why antibiotics kill bacteria without harming your cells.
This field started in the 1800s with Oswald Schmiedeberg, who built the first lab dedicated to studying drugs. Today, it’s the backbone of nearly every FDA-approved medicine. In fact, between 2010 and 2020, 92% of approved drugs came from research rooted in pharmacology. That’s not a coincidence - it’s the result of decades of precise science.
The Two Sides of Drug Action: Pharmacokinetics and Pharmacodynamics
Pharmacology breaks down into two main parts: what your body does to the drug, and what the drug does to your body.
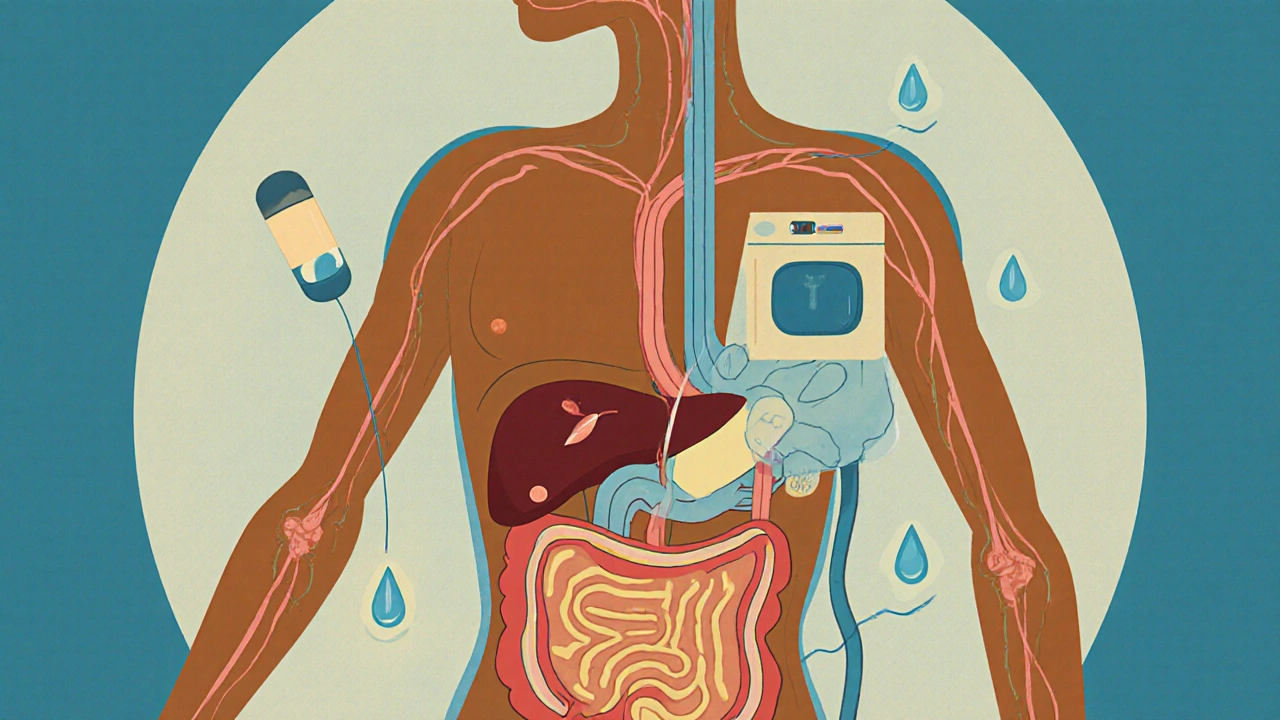
Pharmacokinetics - or PK - tracks the journey of a drug through your body. It’s a four-step process called ADME:
- Absorption: How the drug gets into your bloodstream. A pill swallowed orally must pass through your gut lining. An injection goes straight in. Inhalers deliver drugs through your lungs.
- Distribution: Once in the blood, the drug travels. Some drugs stick to proteins in your blood and move slowly. Others slip easily into tissues like the brain or muscles.
- Metabolism: Your liver breaks down most drugs. Enzymes like CYP450 chop them into smaller pieces so your body can get rid of them. But here’s the catch: your genes control how fast these enzymes work. That’s why two people taking the same dose can have wildly different results.
- Excretion: The leftovers leave through your kidneys (urine), liver (bile), or even your breath. If your kidneys aren’t working well, drugs can build up to dangerous levels.
That’s pharmacokinetics - the movement. Now, pharmacodynamics - or PD - is about the reaction.
How Drugs Actually Change Your Body
Pharmacodynamics answers: How does this drug create its effect? The answer almost always involves proteins - the tiny machines inside your cells.
Most drugs target one of three types of proteins:
- Receptors: These are like locks. Drugs are keys. When a key fits, it turns the lock and triggers a response. About 60% of drugs work this way. Beta-blockers, for example, block adrenaline from binding to heart receptors, slowing your pulse and lowering blood pressure.
- Enzymes: These speed up chemical reactions. Some drugs block them. MAOIs, used for depression, stop the enzyme that breaks down serotonin and dopamine. That raises their levels in your brain, improving mood.
- Transport proteins: These move substances across cell membranes. Diuretics like furosemide block sodium reabsorption in the kidneys, making you pee more and reduce fluid buildup.
Drugs don’t just turn things on or off. They can also:
- Agonists: Activate receptors fully (like morphine on opioid receptors).
- Antagonists: Block receptors without activating them (like naloxone reversing opioid overdoses).
- Partial agonists: Activate receptors weakly (like buprenorphine for opioid addiction).
- Inverse agonists: Do the opposite of what the natural molecule does.
Not all drugs work through proteins. Some act physically. Magnesium citrate, for example, pulls water into your intestines - no receptors needed. It’s an osmotic laxative. It doesn’t trigger a signal; it just changes the environment.

MoA vs. MOA: Why the Difference Matters
People mix up mode of action and mechanism of action. But they’re not the same.
Mechanism of action (MOA) is the molecular-level interaction. For example: “Sertraline blocks the serotonin transporter, increasing serotonin in the brain.”
Mode of action (MoA) is the bigger picture result. “Increased serotonin improves mood and reduces anxiety.”
This distinction matters because two drugs can have the same mode of action but different mechanisms. Two different antidepressants might both lift mood (MoA), but one works on serotonin, another on norepinephrine (MOA). That’s why doctors choose one over the other - side effects, interactions, and genetics all play a role.
Biologics: The New Generation of Drugs
Traditional pills and injections are small molecules. But now, we have biologics - large, complex drugs made from living cells. These include monoclonal antibodies like adalimumab (Humira) and rituximab (Rituxan).
Biologics target specific proteins in your immune system. They block TNF-alpha, IL-17, or other signaling molecules that cause inflammation in diseases like rheumatoid arthritis or psoriasis. Between 2015 and 2022, 35% of new FDA-approved drugs were biologics. They’re powerful, but expensive - and they often need injections or infusions because your stomach would break them down if you swallowed them.
Why One Dose Doesn’t Fit All
Here’s the hard truth: your genes, age, liver health, kidney function, and even what you ate today change how a drug works.
For example:
- People with a CYP2C9 gene variant metabolize warfarin (a blood thinner) slowly. A standard dose can cause dangerous bleeding.
- Older adults often have reduced kidney function. A drug cleared by the kidneys - like metformin - can build up and cause toxicity if the dose isn’t lowered.
- Some drugs interact. Taking an SSRI with an MAOI can trigger serotonin syndrome - a life-threatening spike in serotonin.
A 2023 study of over 12,000 patients found that adjusting doses based on kidney and liver function cut adverse drug events by 27%. Yet, only 62% of doctors feel confident explaining these mechanisms to patients. That gap puts people at risk.
What’s New in Pharmacology
Technology is changing how we understand drugs. DeepMind’s AlphaFold 3, released in May 2024, can predict how a drug binds to a protein with 89% accuracy - far better than older tools. This speeds up drug discovery and helps avoid side effects before a drug even reaches trials.
The FDA now accepts real-world data from electronic health records and wearables to refine dosing. New biomarkers help measure kidney function more accurately, especially in older or sick patients.
And the future? Personalized pharmacodynamics. Imagine a test that tells your doctor not just what drug to give you, but exactly how much - based on your unique receptor expression, metabolism speed, and genetic profile. Pilot studies show this approach improves outcomes by 42%.
But there’s a catch. As the science gets more complex, doctors struggle to keep up. The NIH predicts a 25% increase in pharmacology specialists by 2030 just to bridge this gap.
What This Means for You
You don’t need to become a pharmacologist. But understanding the basics helps you ask better questions.
- If your medication isn’t working, ask: “Could my liver or kidneys be affecting how it’s processed?”
- If you have side effects, ask: “Is this because the drug is blocking or activating something specific?”
- If you’re on multiple drugs, ask: “Do any of these interact with each other?”
Pharmacology isn’t just for doctors. It’s for anyone who takes medication. When you know how drugs work, you’re not just a patient - you’re an informed partner in your care.
What’s the difference between pharmacokinetics and pharmacodynamics?
Pharmacokinetics (PK) is what your body does to the drug - how it’s absorbed, distributed, metabolized, and excreted. Pharmacodynamics (PD) is what the drug does to your body - how it interacts with receptors, enzymes, or other molecules to create its effect. PK is about movement; PD is about action.
Why do some people need higher doses of the same medication?
Differences in metabolism, genetics, body weight, liver or kidney function, and even diet can change how quickly a drug is broken down or how strongly it binds to its target. For example, people with certain CYP450 gene variants metabolize drugs slower and may need lower doses to avoid side effects.
Can drugs work without binding to receptors?
Yes. Some drugs work through physical or chemical actions. Magnesium citrate, for example, draws water into the intestines to soften stool - no receptors involved. Antacids like calcium carbonate neutralize stomach acid chemically. These are called non-receptor-mediated mechanisms.
What are biologics, and how are they different from regular pills?
Biologics are large, complex drugs made from living cells - like antibodies or proteins. Unlike small-molecule pills, they can’t be swallowed because stomach acid destroys them. They’re given by injection or IV and target specific immune signals, such as TNF-alpha in autoimmune diseases. They’re more targeted but also more expensive and require special handling.
How does genetics affect how a drug works?
Your genes control how fast your body breaks down drugs. For example, 17% of people have genetic variants in CYP450 enzymes that make them slow or fast metabolizers. Slow metabolizers can build up toxic levels of a drug, while fast metabolizers may not get enough benefit. This is why genetic testing is becoming part of personalized prescribing.
Why do some drugs have narrow therapeutic indexes?
A narrow therapeutic index means the difference between a helpful dose and a dangerous one is very small. Warfarin and digoxin are examples. Small changes in metabolism, diet, or other drugs can push levels into the toxic range. That’s why these drugs require frequent monitoring and careful dosing.
What’s the biggest challenge in pharmacology today?
The biggest challenge is translating complex science into simple, practical care. As drugs get more targeted and personalized, doctors and patients struggle to keep up. The gap between research and real-world prescribing is growing. Solutions include better education, AI tools, and integrating genetic data into electronic health records.
What to Do Next
If you’re on multiple medications, ask your doctor or pharmacist for a medication review. Bring a list - including supplements and over-the-counter drugs. Ask about interactions, side effects, and whether your dose still makes sense based on your current health.
If you’re a student or just curious, explore free resources like the Pharmacology Education Project or NCBI’s open-access textbooks. Understanding how drugs work doesn’t just help you - it helps everyone around you.
Medications aren’t magic bullets. They’re precise tools. And like any tool, they work best when you know how to use them.




Shante Ajadeen
12 November 2025 - 05:23 AM
Love this breakdown. I used to just swallow pills and hope for the best, but now I ask my pharmacist about PK and PD like it’s a Netflix show. Turns out my body’s just a weird chemistry lab with bad Wi-Fi.
Erica Cruz
12 November 2025 - 10:02 AM
Ugh. Another ‘pharmacology is cool’ post. Everyone acts like this is groundbreaking. I’ve seen this exact diagram in med school textbooks from 2008. The real story? Pharma companies pay researchers to make this stuff sound like science when it’s just profit-driven guesswork with a lab coat.
Johnson Abraham
13 November 2025 - 10:00 AM
lol so drugs are just keys and locks? then why does my adderall make me feel like a robot but my friend’s same dose makes him cry? 🤔 maybe the lock is broken??
dace yates
14 November 2025 - 12:52 PM
Wait, so if CYP450 variants affect metabolism, does that mean some people are just naturally ‘drug-resistant’ because of their genes? Like, is that why my grandma’s blood pressure med doesn’t work but mine does?
Danae Miley
16 November 2025 - 04:44 AM
Incorrect terminology used in the post: ‘mode of action’ and ‘mechanism of action’ are often conflated, but in clinical pharmacology, MOA is the accepted shorthand for mechanism of action. Mode of action refers to physiological effects, not molecular interactions. Please correct this before misleading patients.
Charles Lewis
16 November 2025 - 06:41 AM
It is imperative to recognize that the evolution of pharmacological understanding over the past century has not only transformed clinical outcomes but has also illuminated the profound interplay between genetic variability and therapeutic efficacy. The notion that a ‘one-size-fits-all’ dosing paradigm remains viable in the 21st century is not merely outdated-it is ethically indefensible. We must integrate pharmacogenomics into routine practice, not as an experimental adjunct, but as a foundational pillar of patient-centered care.
Renee Ruth
16 November 2025 - 17:53 PM
AlphaFold 3? Yeah right. Big Pharma is using AI to hide the fact that 90% of these drugs are just repackaged toxins. They don’t care if you live or die-only if your insurance pays. I’ve seen people die from ‘safe’ meds. This isn’t science. It’s corporate theater.
Deepa Lakshminarasimhan
18 November 2025 - 01:37 AM
so uhh... if my genes make me a slow metabolizer, does that mean my body is secretly sabotaging me? like... am i genetically programmed to need less meds or is this just another way for pharma to sell more tests? i dont trust any of this. why do i feel like i'm being tested before i even take a pill?
Samantha Wade
19 November 2025 - 02:17 AM
Charles, you’re absolutely right. The gap between research and practice is a crisis. But we can fix it. Hospitals need embedded pharmacogenomics teams. EHRs must auto-flag high-risk interactions. And patients? We need plain-language handouts-not PDFs buried in portals. This isn’t science fiction. It’s a moral imperative.
Elizabeth Buján
20 November 2025 - 16:27 PM
it’s wild to think that a tiny molecule can change how you feel for days… like your whole mood, your energy, even your dreams. we take these things so lightly. but really, we’re letting chemicals whisper into our brains and hearts. it’s kinda beautiful. and kinda terrifying. 🤯
Andrew Forthmuller
21 November 2025 - 07:56 AM
so biologics = injections only? why cant we just make them pills? smh
vanessa k
23 November 2025 - 00:29 AM
thank you for writing this. i’ve been on 7 meds for 5 years and no one ever explained how they actually work. now i feel less like a guinea pig and more like someone who’s trying to understand their own body. i’m not alone in this.
manish kumar
24 November 2025 - 20:10 PM
I’ve been a pharmacist in Mumbai for 18 years and let me tell you, the real issue isn’t just genetics or AI-it’s access. In India, 80% of patients can’t afford even basic pharmacogenetic testing. We give the same dose to a 50kg woman in Delhi and a 90kg man in Bangalore because we have no choice. The science is brilliant, but the system is broken. We need affordable screening, not just fancy tools. Real change starts with equity, not just algorithms.